p300/CBP Inhibitor A-485 Suppresses Osteoclast Differentiation and Mitigates Osteoporotic Bone Loss
Osteoporosis is a prevalent skeletal disorder driven by an imbalance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption, with excessive osteoclast activity being the dominant factor. Osteoclasts, the sole cells capable of bone resorption, are giant multinucleated cells derived from hematopoietic cells of the monocyte/macrophage lineage. Their maturation relies on the presence of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL).
In osteoclast precursors, M-CSF binds to its receptor (CSF receptor 1, cFMS) to regulate cell proliferation and survival during differentiation. RANKL, a member of the tumor necrosis factor (TNF) superfamily, is responsible for osteoclast maturation and resorption. By binding to RANK, RANKL activates multiple signaling cascades, including the NF-κB, MAPK, and PI3K-Akt pathways, which initiate the differentiation and fusion of osteoclast precursors into mature osteoclasts. These pathways synergistically activate or induce the expression of key osteoclastogenic transcription factors, such as tartrate-resistant acid phosphatase (TRAP), nuclear factor-activated T cells c1 (NFATc1), cathepsin K, and c-Fos. Therefore, inhibitors targeting these pathways hold potential for osteoporosis treatment.
In recent years, molecular research on the crosstalk between osteoclastic bone resorption and osteoblastic bone formation has led to the development of several drugs for clinical prevention and treatment of osteoporosis, including bisphosphonates (BPs), teriparatide (PTH), denosumab, and selective estrogen receptor modulators. However, most current treatments are associated with severe adverse effects that limit long-term administration and patient adherence. Thus, developing novel therapies with enhanced efficacy and reduced toxicity is crucial.
CREB (cyclic-AMP response element binding protein) binding protein (CBP) and E1A binding protein (p300) are highly homologous proteins with 96% sequence similarity, predominantly expressed in humans and eukaryotes. As transcriptional co-activators, they integrate and maintain various gene regulatory pathways and protein acetylation events through their intrinsic histone acetyltransferase (HAT) activity. Recent studies have also highlighted their pivotal roles in differentiation, apoptosis, and the cell cycle. Consequently, CBP/p300 inhibitors have emerged as promising epigenetic targets for diverse human diseases, including inflammation, cancer, autoimmune disorders, and cardiovascular disease. However, their impact on bone metabolism remains unclear.
In this study, we explored whether A-485, a highly selective catalytic p300/CBP inhibitor, could attenuate RANKL-induced osteoclast differentiation and investigated the underlying molecular mechanisms.
A-485 Treatment Exhibits No Cytotoxicity to Cells
We first evaluated the effects of A-485 on bone marrow-derived macrophages (BMMs), rat bone marrow mesenchymal stem cells (rBMSCs), and MC-3 T3 cells. Cells were cultured with different concentrations of A-485, and cell cytotoxicity was assessed using a CCK-8 assay. The range of cytotoxic responses varied across cell types.
A-485 did not significantly inhibit the growth of all three cell types at concentrations up to 5 μM, even when treatment duration was extended to 5 days. For BMMs, cell viability was not significantly affected by A-485 at concentrations up to 50 μM on day 1, and up to 20 μM on days 3 and 5. For rBMSCs, cytotoxicity was observed after treatment with 10 μM A-485 on the first day of cultivation. For MC-3 T3 cells, no significant differences in cell viability were observed compared to the control group, except at 20 μM where viability was inhibited. These results indicate that A-485 is a nontoxic agent for mammalian cells.
A-485 Inhibits RANKL-Induced Osteoclast Differentiation
To determine whether A-485 inhibits RANKL-induced osteoclastogenesis, we stained BMMs for TRAP, a specific marker of mature osteoclasts. A-485 inhibited mature osteoclast formation in a dose-dependent manner, as evidenced by a reduction in the number of TRAP-positive multinucleated (3 nuclei) osteoclasts across a range of concentrations. Additionally, A-485 treatment significantly decreased the number of multinucleated osteoclasts compared to the control group in a dose-dependent manner.
Mature osteoclasts were also stained with rhodamine phalloidin to visualize F-actin rings, another characteristic feature necessary for bone resorption. Well-defined actin rings were observed in the control group, while A-485 clearly suppressed osteoclast and F-actin ring formation. We further examined the effect of A-485 on osteoclastic bone resorption activity using devitalized bovine bone discs. Numerous resorption pits were observed in RANKL-stimulated cells (indicated by yellow arrows), fewer pits were present in A-485-treated cells, and almost no pits were detected in the 2 µM A-485 group. Collectively, these results demonstrate that A-485 significantly inhibits RANKL-induced osteoclast formation and osteoclastic bone resorption activity in vitro.
A-485 Attenuates Osteoclast-Specific Gene Expression
RANKL stimulation is known to upregulate several genes associated with osteoclast differentiation. We assessed the expression of osteoclast-specific genes, including CTSK, DC-STAMP, c-Fos, VATPs-d2, NFATc1, and TRAP, using real-time RT-PCR. A-485 dramatically suppressed the expression of these genes in a time- and dose-dependent manner, suggesting that it inhibits osteoclast differentiation by downregulating RANKL-induced gene expression.
A-485 Suppresses MAPK and NFATc1 Activation
To elucidate the underlying molecular mechanisms of A-485's anti-osteoclastogenic role, we investigated the activation of the MAPK signaling pathway, which is closely linked to osteoclast differentiation, using western blotting. After A-485 treatment, phosphorylation of ERK, JNK, and P38 was clearly downregulated compared to the RANKL-induced group, indicating impaired MAPK pathway activation.
We next blocked the MAPK signaling pathways using specific inhibitors: ERK inhibitor (U0126), JNK inhibitor (SP600125), and P38 inhibitor (SB203580). Western blot analysis showed that the inhibitory effect of A-485 on the phosphorylation of ERK, JNK, and P38 proteins was comparable to that of their respective inhibitors. Quantified western blot results are presented separately.
NFATc1 is a critical transcription factor regarded as a master switch for osteoclast differentiation and maturation, making it a potential target of A-485. NFATc1 induction was dramatically upregulated after 3 and 5 days of RANKL treatment, but was significantly attenuated in the presence of A-485. This finding was consistent with real-time PCR results, which showed that NFATc1 mRNA expression was reduced by A-485 in a time- and dose-dependent manner. These results strongly suggest that A-485 inhibits MAPK and NFATc1 activation during osteoclastogenesis.
A-485 Prevents OVX-Induced Osteoporosis
Given the promising in vitro effects of A-485, we investigated its role in ovariectomized (OVX)-induced bone loss using an established mouse model of osteoporosis. Micro-CT imaging was performed to examine bone loss in the distal femur. As expected, OVX led to extensive trabecular bone loss in the vehicle group, with bone density-related parameters (BMD, BV/TV, Tb.Th, and Tb.N) significantly decreased and Tb.Sp and SMI increased compared to the sham-operated control group.
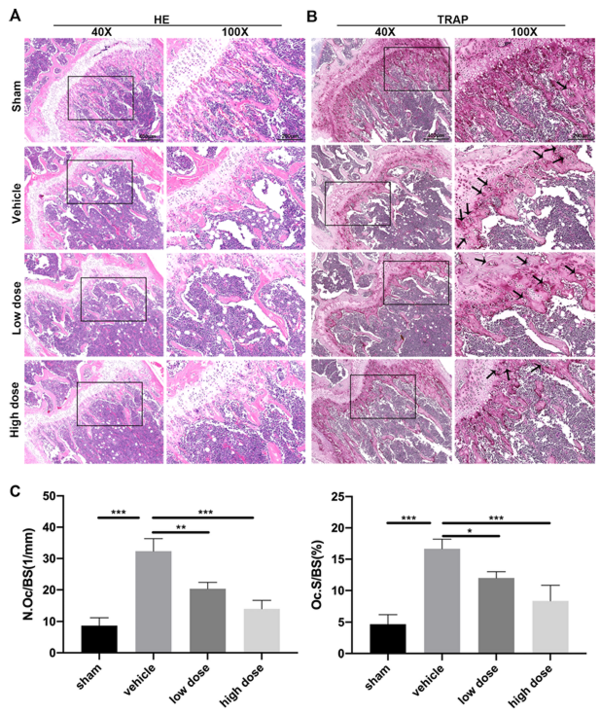
A-485 significantly reversed these changes. Histological analysis confirmed that A-485 treatment significantly reduced OVX-induced bone mass loss compared to the untreated group. This improvement was attributed to decreased osteoclast activity, as evidenced by a reduced number of TRAP-positive osteoclasts in the bone. These in vivo findings indicate that A-485 can reduce bone loss and may serve as a useful therapeutic agent for osteoporosis.
 Summary
Summary
Osteoporosis is the most common bone disease, with prevalence increasing with age and decreasing bone density. It varies by gender, race, and ethnicity. Postmenopausal women are at particularly high risk due to rapid bone loss caused by reduced ovarian estrogen production after menopause. Decreased bone mass and disrupted bone microarchitecture, including trabecular loss, lead to skeletal fragility, diminished bone strength, and increased fracture risk. Due to its association with age-related fractures that elevate morbidity and mortality, osteoporosis imposes a significant clinical and public health burden.
Although drugs such as BPs, calcitonin, estrogen therapy, and RANKL inhibitors have been developed for osteoporosis treatment, alternative approaches are needed due to their severe side effects. Bisphosphonates, the most widely prescribed agents for osteoporosis management, may cause osteonecrosis of the jaw in some patients. Thus, more experimental research is focused on developing tolerable and multifunctional agents that alleviate pain and prevent fractures in elderly women.
CBP and p300 are highly homologous transcriptional co-activators with 96% sequence similarity, mainly expressed in humans and eukaryotes. They integrate and maintain various gene regulatory pathways and protein acetylation events through their intrinsic HAT activity. Numerous studies have demonstrated their crucial roles in differentiation, apoptosis, and the cell cycle. A-485 is a potent and selective catalytic inhibitor of p300/CBP, with IC50 values of 9.8 and 2.6 nM against the HAT activities of p300 and CBP, respectively. While its anti-inflammatory, anti-oxidative, and anti-tumor effects have been well characterized, its impact on bone metabolism has not been previously described.
In this study, we verified the anti-osteoporotic effects of A-485 both in vitro and in vivo. CCK8 assays confirmed the safety of A-485, as it did not alter BMM viability over the concentration range that inhibited osteoclast differentiation. TRAP staining showed that A-485 significantly reduced the number of multinucleated cells in a dose-dependent manner. Resorption pit assays and F-actin ring staining further confirmed that A-485 inhibits RANKL-induced osteoclastogenesis. Western blotting revealed that A-485 significantly downregulated critical regulatory genes for osteoclast differentiation, including NFATc1, c-Fos, and CTSK.
MAPK signaling pathways are major mediators of inflammatory lesions and play crucial roles in osteoclast activation. It has been reported that A-485 can reduce the inflammatory response by inhibiting the MAPK pathway in macrophages. Consistently, our western blot data showed that A-485 inhibited p38, JNK, and ERK phosphorylation, thereby reducing osteoclast activation. These findings suggest that A-485 dramatically inhibits osteoclast function and differentiation in vitro and could be a potential therapeutic option for osteoporosis.
To confirm the protective role of A-485 in osteoporosis, we conducted in vivo experiments using an OVX-induced bone loss mouse model. A-485 attenuated OVX-induced bone loss caused by low estrogen levels and significantly increased trabecular bone quantity. Quantitative micro-CT analysis revealed that A-485 significantly rescued OVX-induced loss of BMD and BV/TV. Thus, our in vivo findings confirm that A-485 inhibits osteoclastogenesis.
Despite the extensive work and demonstrated protective effects of A-485 in this study, the detailed mechanisms by which A-485 inhibits osteoclast differentiation remain unclear. This prompts further investigation into the specific association between A-485 and bone formation, with efforts to control confounding factors such as reduced physical activity or presence of inflammatory conditions.